Share:

Abstract
This paper uses a 1064nm picosecond laser with a microlens array (MLA) and a diffractive optical element (DOE) to evaluate the correlation of laser-induced optical breakdown (LIOB) with skin type. The LIOB effect of laser-induced skin cavitation was evaluated by examining black and white skin tissues. Under irradiation with an energy density of 3.0 J/cm2, black skin showed a significant difference compared to DOE (180- 280μm; 40μm), MLA produced laser-induced cavitation deeper (180-400μm), with a size of 67μm. However, compared with MLA in black skin, MLA in white skin was deeper (125-700μm) and produced The cavitation was larger (134 μm) and less numerous. In white skin, DOE did not produce laser-induced cavitation. White skin tissue with higher scattering may result in deeper cavitation after picosecond laser treatment. Research is expected to determine the optimal treatment conditions for various skin types.
Keywords: Diffractive optical element, Fitzpatrick skin type, Laser-induced optics Breakdown, microlens array, picosecond laser treatment
Introduction
Melasma (occurrence During pregnancy, it is a common pigmentary disorder that is caused by excessive pigmentation in the epidermis and dermis of the skin. This disease often occurs on prominent areas of the face exposed to the sun, such as the cheeks, upper lip, and forehead. Melasma occurs in all people and is usually accompanied by darker spots (brown, grey and black) on the skin. Melasma can be caused by: ultraviolet (UV) rays, accumulation of melanin in the skin, pregnancy, genetics, and hormonal imbalance, etc. However, the exact pathogenesis of melasma is still unclear. Melasma mainly occurs in Fitzpatrick types IV-VI skin, because the Fitzpatrick scale is based on the number of melanocytes in the skin and the skin's response to sunlight exposure. Type classification system. However, due to the higher sensitivity of Fitzpatrick I-III skin to UV rays, it is also prone to melasma.
Picosecond laser treatment is often used to treat pigmented skin diseases due to its non-invasiveness and short recovery time. Picosecond laser has a pulse width of Within a few hundred picoseconds, high pressure (2GPa) and high temperature (2000-4000K) are generated through multiphoton ionization in the skin. In addition, both high pressure and high temperature cause the plasma to expand and generate shock waves, which lead to cavitation inside the skin. ization, which is called laser-induced optical breakdown (LIOB). Laser-induced cavitation caused by LIOB destroys melanin in the epidermis and dermis without causing or minimizing thermal damage, while inducing collagen regeneration within the cavitation bubble and restoring the skin condition. Unnecessary thermal damage, clinically using microlens arrays (MLA) and diffractive optical elements (DOE) with picosecond laser systems, MLA has a Gaussian-like microbeam distribution, while DOE has a flat-like microbeam distribution. Due to the inherent optical properties, any lens needs to be selected according to the condition of melasma. For example, MLA is used to treat dark melasma located in the dermis, while DOE is used to remove light melasma that is widely distributed in the skin. Despite successful melasma removal, clinical results of picosecond laser treatments often depend on skin type.
This study aims to compare and evaluate the Correlation of the LIOB effect with two different skin types (Fitzpatrick I and VI skin types) after irradiation with 1064 nm picosecond laser with MLA and DOE under (H0, J/cm2). We assume that under the same conditions, Dark skin produces larger cavitation in the deep dermis compared to light skin with low melanin index and high scattering. Two ex vivo porcine skin tissues (black) were tested using a 1064nm picosecond laser system with MLA and DOE. and white) to compare the distribution and location of laser-induced cavitation in the skin layer. Histological analysis was performed to quantitatively evaluate the depth, size, and distribution of laser-induced cavitation under the basement membrane.
Experiment
2.1 Light Source
In LIOB experiment, 1064nm Nd:YAG The second laser system (pulse width = 450 ps at full width at half maximum [FFWH]; Picore, Bluecore, Busan, South Korea) and the MLA (fused silica, circular overall beam diameter = 4 mm, microbeam diameter = 220 μm, 37 microbeams) were used. , focal length = 40 mm; Bluecore, Busan, South Korea) and DOE (fused silica, rectangular overall beam size = 4 mm, microbeam diameter = 165 μm, 49 microbeams, focal length = 40 mm; Bluecore, Busan, South Korea) were used together. MLA and DOE were used to grade the incident overall beam to maximize skin cavitation after laser-induced LIOB. Two energy densities (H0 = 3.0 and 6.0 J/cm2) were used for MLA and DOE to compare different To provide equal energy density to the target tissue, the microbeam energy of MLA was 10.3 and 20.5 mJ/microbeam (total beam energy/pulse = 0.38 and 0.76 J), and the microbeam energy of DOE was 10.3 and 20.5 mJ/microbeam (total beam energy/pulse = 0.38 and 0.76 J). The beam energies were 9.8 and 19.6 mJ/microbeam (total beam energy/pulse = 0.48 and 0.96 J). According to preliminary studies, in order to produce laser-induced cavitation under the basement membrane while minimizing damage to the tissue surface, The focal depth was set to 35 mm (from the lens to the target tissue). MLA and DOE irradiated black dimming paper with a laser energy density of 3.0 J/cm2, and the spatial distribution of the beam was compared by evaluating the power density of a single microbeam.
2.2 In vitro experiments
Results
Figure 1 shows the laser-induced response of ex vivo porcine skin tissue to MLA and DOE laser irradiation. Figure 1A shows the laser-induced response of ex vivo porcine skin tissue to MLA and DOE laser irradiation. CIE Lab color space distribution of Fitzpatrick skin type determined by the Luschan scale. The CIE Lab color values of Fitzpatrick skin type are widely distributed (L*=13-96, a*=-3-18, b*=-6-39). The CIE Lab color value of black skin is L*=29, a*=4.4, b*=7.4, corresponding to Fitzpatrick V and VI skin types (L*=13-28, a*=5.7-18, b*=- 6-20). On the other hand, white skin has L*=70, a*=2.5, b*=7.8, representing Fitzpatrick I and II skin types (L*=96.4~96.5, a*=-0.4-0.03, b*=4.8- 5.9). It can be seen that the L*, a*, and b* values of white skin are lower than those of Fitzpatrick skin type, which may be due to the lack of blood flow after tissue extraction. Figure 1B shows the results of MLA and DOE at 3.0 J/ Top view of the black and white skin surface after 20 irradiations with 6.0 J/cm2 (top) and 6.0 J/cm2 (bottom). MLA produces a circular profile beam, while DOE produces a rectangular profile beam. Figure 1C shows the MLA and DOE on black dimming paper The beam distribution of MLA is relatively large, and the light intensity is higher at the center. On the other hand, DOE produces a relatively uniform beam distribution. Due to the strong absorption of light, black skin can clearly show the overlapping overall beam spots regardless of the type of lens. On the other hand, white skin shows blurred or less obvious overall beam spots and traces of thermal damage after MLA and DOE irradiation.

Figure 1. Comparison of ex vivo porcine skin tissues of different skin colors (black and white) after irradiation with microlens array (MLA) and diffractive optical element (DOE) at H0=3.0 and 6.0J/cm2: (A) CIE of Fitzpatrick I–VI skin types and the tested skin tissues (circles) Lab color space value (A*=red/green; b*=blue/yellow; L*=perceived brightness) distribution, (B) top view of laser-irradiated skin, and (C) microbeam images and beam distribution of MLA and DOE at 3.0 J/cm2 (bar=1 mm). It is worth noting that the overall beam moved from left (orange dashed line) to right (red dashed line) during irradiation (lateral movement=50 μm/pulse; 20 irradiations in total; bar=2 mm; green bar=1 mm)
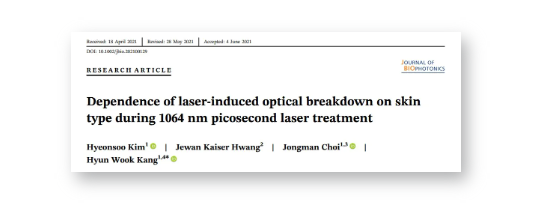
Figure 2 shows HE staining images of black and white skin tissues after laser irradiation with MLA and DOE at H0=3.0 J/cm2. In black skin, MLA and DOE can obviously produce laser-induced vacuoles under the basement membrane. According to Figure 2A, MLA produced multiple small vacuoles in the depth from the papillary dermis to the reticular dermis in black skin. Larger and deeper vacuoles were observed in white skin compared to black skin (vacuolar depth = 283 ± 114 μm in black skin and 368 ± 171 μm in white skin; P<.01, vacuolar size = 67 ± 34 μm in black skin and 134 ± 100 μm in white skin; P<.001; Figure 2A). On the other hand, DOE induced relatively fewer vacuoles near the papillary dermis in black skin compared to MLA (vacuolar depth = 140 ± 62 μm; Figure 2B). However, white skin did not show vacuolated photodestructive damage in the papillary dermis.
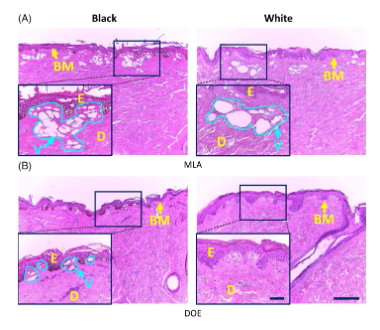
Figure 2. Histological images (HE staining) of black (left column) and white (right column) porcine skin tissue after irradiation with (A) microlens array (MLA) and (B) diffractive optical element (DOE) at H0=3.0J/cm2 (100 and bar=200μm). Note that the inlet (400 and bar=50μm) indicates the magnified area of the skin surface (solid black line; E: epidermis, D: dermis, BM: basement membrane, V: laser-induced vacuoles)
Figure 3 shows the HE staining images of black and white skin tissues after laser irradiation with MLA and DOE at H0=6.0 J/cm2. For black and white skin tissues, MLA and DOE produced vacuoles under the basement membrane. In Figure 3A, the depth distribution of vacuoles produced by MLA in black and white skin tissues. However, white skin underwent photoablation in the upper epidermis and produced larger vacuoles than black skin (i.e., black vacuoles were 70±37μm in size and white vacuoles were 162±98μm in size; P<0.001). In Figure 3B, DOE produced laser-induced cavitation near the papillary dermis in black skin (cavitation depth = 210 ± 54 μm), but the size was smaller than that in MLA (P<0.001). However, no laser-induced cavitation was found in white skin after DOE irradiation.
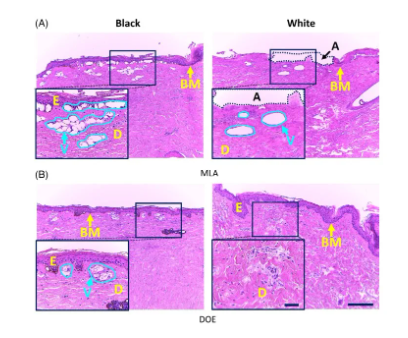
Figure 3. Histological images (HE staining) of black (left column) and white (right column) porcine skin tissue after irradiation with (A) microlens array (MLA) and (B) diffractive optical element (DOE) at H0=6.0J/cm2 (100 and bar=200μm). It is worth noting that the inlet (400 and bar = 50 μm) represents the magnified area of the skin surface (solid black line; E: epidermis, D: dermis, BM: basement membrane, V: laser-induced cavitation)
Figure 4 compares the number of laser-induced cavitation in black and white skin tissues at different cavitation depths and H0. Figure 4A shows that MLA produces more cavitations and a deeper range of cavitation (160 to 550 μm) compared to DOE (160 to 280 μm) regardless of skin type. For black skin, MLA and DOE at 3.0 J/cm2 produced the highest number of cavitations at a depth of ~200 μm. On the other hand, after MLA laser irradiation, white skin produced deeper cavitation than black skin (~200μm in black skin and ~380μm in white skin), but the number of cavitations was relatively small (~110 in black skin and ~60 in white skin; P<0.05; Figure 4A). However, after DOE irradiation of white skin, no or very small cavitations were observed. When H0=6.0J/cm2, the number of cavitations produced by MLA and DOE on black skin was the highest at a depth of 200μm (Figure 4B). Higher H0 resulted in significant changes in the mean cavitation depth (cavitation depth in black skin, 3.0J/cm2 cavitation depth = 283±114μm, 6.0J/cm2 cavitation depth = 294±126μm; P=.50). On the other hand, white skin produced fewer cavitations after irradiation with MLA and DOE.
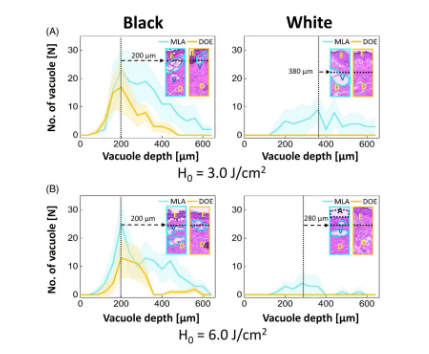
Figure 4. Quantitative analysis of black and white skin tissue measured from histological images after irradiation with microlens array (MLA) and diffractive optical element (DOE): comparison of the depth and number of cavitation bubbles generated at (A) H0=3.0J/cm2 and (B) H0=6.0J/cm2. It is worth noting that the inlet (100) indicates the distribution of vacuoles in the tissue (E: epidermis, D: dermis, V: laser-induced vacuoles, A: ablation area; filling area around the line: SD) Figure 5 quantifies the function of vacuoles size in black and white skin tissues after irradiation with MLA and DOE at H0=3.0 and 6.0J/cm2. According to Figure 5A, MLA and DOE at H0=3.0J/cm2 produced the most vacuoles in black skin, with a size of 70μm. MLA produced more vacuoles than DOE (~170 for MLA and ~70 for DOE; P<0.05). On the other hand, fewer but larger vacuoles were found under the basement membrane of white skin after MLA irradiation compared to black skin (mean size = 134±100μm) (Figure 5A). DOE induced photodamage in the papillary dermis without laser-induced cavitation. Figure 5B shows that despite the higher H0, MLA and DOE produced fewer laser-induced cavitations of 55-70 μm in black skin. MLA produced more cavitations than DOE (P<0.05). On the other hand, MLA produced a smaller number of large cavitations (cavitation size = 162 ± 98 μm) in white skin. DOE did not produce laser-induced cavitation in white skin with photodamage of the dermis.
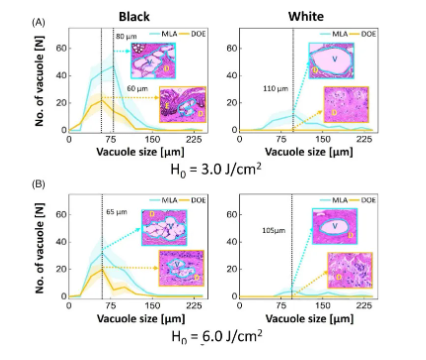
Figure 5. Quantitative analysis of black and white skin tissue measured from histological images after irradiation with microlens array (MLA) and diffractive optical element (DOE): comparison of the size and number of cavitation bubbles generated at (A) H0=3.0J/cm2 and (B) H0=6.0J/cm2. It is worth noting that the inlet (400) represents the average cavitation size (E: epidermis, D: dermis, V: laser-induced cavitation bubble; filling area around the line: SD)
Figure 6 shows the variation of laser-induced cavitation coverage with skin tissue depth after MLA and DOE irradiation of black and white skin tissue under H0=3.0 and 6.0J/cm2 conditions. The cavitation coverage represents the relative spatial distribution of cavitation generated in the target area of the skin. In Figure 6A, regardless of any skin type, MLA and DOE generated a large number of cavitations under the basement membrane at H0=3.0J/cm2. In black skin, the cavitation coverage of MLA is deeper (180-400μm), while the coverage of DOE is relatively narrow (180-280μm). MLA and DOE produced maximum coverage of 22% and 17% at 360 μm and 260 μm, respectively. The average coverage was 14% for MLA and 6% for DOE. In white skin, MLA produced a small number of cavitations in a deeper distribution (125-700 μm) relative to black skin. The maximum cavitation coverage was 22% and occurred at 460 μm (average coverage was 15%). On the other hand, DOE did not produce laser-induced cavitation in white skin (coverage was 0%). Figure 6B shows the cavitation coverage after irradiation with MLA and DOE at H0 = 6.0 J/cm2. In black skin, MLA produced a deeper distribution of cavitations than DOE (i.e., 180-550 μm for MLA and 225-400 μm for DOE). MLA produced the maximum cavitation coverage of 24% at 360 μm, while DOE produced the maximum coverage of 14% at 300 μm. The average coverage of MLA and DOE was 13.5% and 5.3%, respectively, which was higher than that at H0=3.0J/cm2. For white skin, MLA showed photoablation in the epidermis and papillary dermis, resulting in fewer cavitations in a narrower distribution (125-750μm) in white skin than under irradiation with H0=3.0J/cm2 (Figure 3A). The maximum cavitation coverage was 21%, occurring at 375μm (average coverage = 10%). However, DOE still did not cause laser-induced cavitation in white skin (0% coverage). Table 1 summarizes the correlation of laser-induced cavitation with skin color, energy density, and lens type.
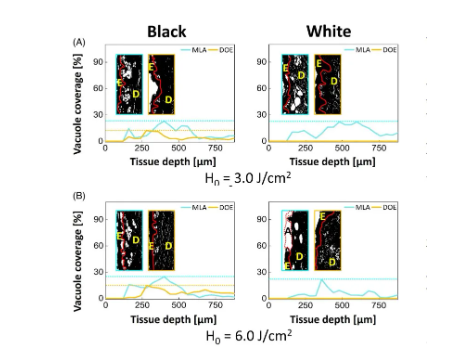
Figure 6. Quantitative comparison of the variation of cavitation coverage with depth after irradiation of black and white skin tissue with microlens array (MLA) and diffractive optical element (DOE) under (A) H0=3.0J/cm2 and (B) H0=6.0J/cm2. It is worth noting that the entrance (100) represents the binary image classification of cavitation in tissue (E: epidermis , D: dermis, red line: basement membrane, A: ablation area)
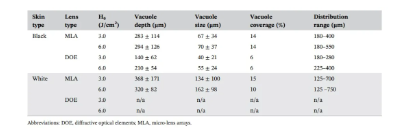
Table 1. Correlation of laser-induced cavitation with skin type, lens type and H0
Discussion
Treatment of melasma has been extensively studied due to its high prevalence in all populations. Although current clinical treatments focus on the location, size, and depth of melasma, treatment conditions need to be optimized for skin containing different types of melanin. The aim of the current study was to evaluate the relevance of the LIOB effect to two different skin types (black and white) by using a 1064 nm picosecond laser with MLA and DOE. The CIE Lab color space classified the tested black and white skin tissues as Fitzpatrick VI and I skin types. Due to the high prevalence of the disease in all populations, ex vivo porcine skin tissues differ from human skin properties in terms of blood flow, temperature, and water content after tissue extraction. However, ex vivo skin was able to show values appropriate for the Fitzpatrick skin type. Black skin showed laser-induced cavitation near the basement membrane (depth = 283 μm for MLA and depth = 140 μm for DOE), and the size of the vacuoles formed was relatively uniform (e.g., 67 μm for MLA and 40 μm for DOE at 3.0 J/cm2). On the other hand, white skin irradiated with MLA produced larger cavitation at a deeper location, but with a smaller number, which may be caused by the high scattering characteristics (depth = 368μm, size = 134μm for MLA at 3.0J/cm2; Figures 4-6). It is worth noting that the DOE with a smaller microbeam energy did not produce laser-induced cavitation. Figure 6 shows that there was no significant change in the average cavitation coverage between the different skin types, but the changes in the number, size, and distribution of cavitation were obvious (Figures 4 and 5). The coverage between the two skin types was comparable, which means that white skin produced larger cavitation than black skin, but with a smaller number. It is conceivable that black skin with a higher melanin content can absorb light immediately and produce LIOB in the tissue, thus forming cavitation in the skin. In contrast, white skin has relatively less light absorption due to its high specular reflection and scattering, which ultimately leads to weaker LIOB in the tissue. If a higher energy density is used to increase the LIOB effect, photodamage will occur in the epidermis and papillary dermis (Figure 3).
Recently, many studies have demonstrated the effects of LIOB after picosecond laser treatment on different skin types. Moustafa et al. examined Fitzpatrick skin types III and IV using 1064 and 532 nm picosecond lasers (H0=4 J/cm2). Their results showed that after the first treatment, the rate of pigmentation clearance was 100% in patients with skin type III and 75% in patients with skin type IV at the 11th to 16th week follow-up. The larger vacuoles generated in white skin (Figures 2, 3, and 5) can lead to more effective pigment removal as well as collagen regeneration compared to black skin. Nguyen et al. removed pigmented lesions in Fitzpatrick skin types III to IV by using 1064 nm laser with H0=1.05 to 1.12 J/cm2. Pigmentation removal rates range from 75% to 90% in skin types III to IV, respectively, and the melanin index in the skin plays an important role in generating photodisruption. The lower melanin index and high scattering characteristics in white skin may result in a wider spatial distribution of incident photons, thereby expanding laser-induced cavitation. Large cavitation can effectively remove pigment from the skin. However, large cavitation may take longer to heal than small cavitation due to collagen remodeling. Therefore, a small number of laser pulses can be applied to white skin to reduce the size of cavitation while having an equivalent LIOB effect.
This study compared the LIOB effect by testing two different colors of ex vivo porcine skin. Although white skin experimentally showed larger and deeper laser-induced cavitation than black skin, it has different optical properties (absorption and scattering) from human skin tissue due to the absence of blood flow, moisture, and constant skin temperature. Therefore, the inhomogeneous optical properties of human skin tissue can change the extent of the LIOB effect during 1064nm picosecond laser treatment. In addition, this study compared the response of two different skin types to picosecond laser treatment by quantifying the CIE Lab color value of skin color and the extent of laser-induced cavitation. Compared with MLA, DOE showed no cavitation in white skin regardless of the energy density, which may be due to the lower microbeam energy level. Therefore, various irradiation conditions, such as overall beam size, focal depth, and energy density, should be optimized to maximize laser-induced cavitation in tissue. For clinical translation, further studies are needed to evaluate the relevance of LIOB in human skin types in terms of recovery time and melasma clearance.
Conclusion
This study demonstrated the spatial correlation of LIOB on different ex vivo porcine skin types by using MLA and DOE-assisted 1064nm picosecond laser. Compared with black skin, the high scattering and less surface light absorption of white skin may lead to a decrease in the number of laser-induced cavitation at deeper locations. Further studies will verify the results of this study on different human skin types to ensure the effectiveness and safety of clinical applications.
Foremed Legend
The core founding team of Suzhou Foremed Legend Medical Technology Co., Ltd. comes from well-known universities at home and abroad such as Peking University. Foremed Legend focuses on the design, development and application of high-end medical beauty optoelectronic equipment based on compliance and product strength, and is committed to becoming a leading company in the field of high-end medical beauty optoelectronic equipment, a provider of integrated intelligent solutions for diagnosis and treatment, and a pioneer of medical beauty data integration platform.
Through tackling a series of underlying key technologies, Foremed Legend has independently developed a number of high-end medical equipment such as picosecond laser therapy, long pulse laser therapy, intense pulse light therapy, photoacoustic imaging skin detection equipment and cold air therapy, and continues to deepen the research and development of core product technologies, using better technical solutions to benefit the vast number of beauty seekers.
Adhering to the principle of science and technology for good, Foremed Legend will work with industry and ecological partners to bring more safe and effective medical beauty optoelectronic equipment and integrated diagnosis and treatment solutions to the global medical beauty market.
Copyright © Suzhou Foremed Legend Technology Co., Ltd.